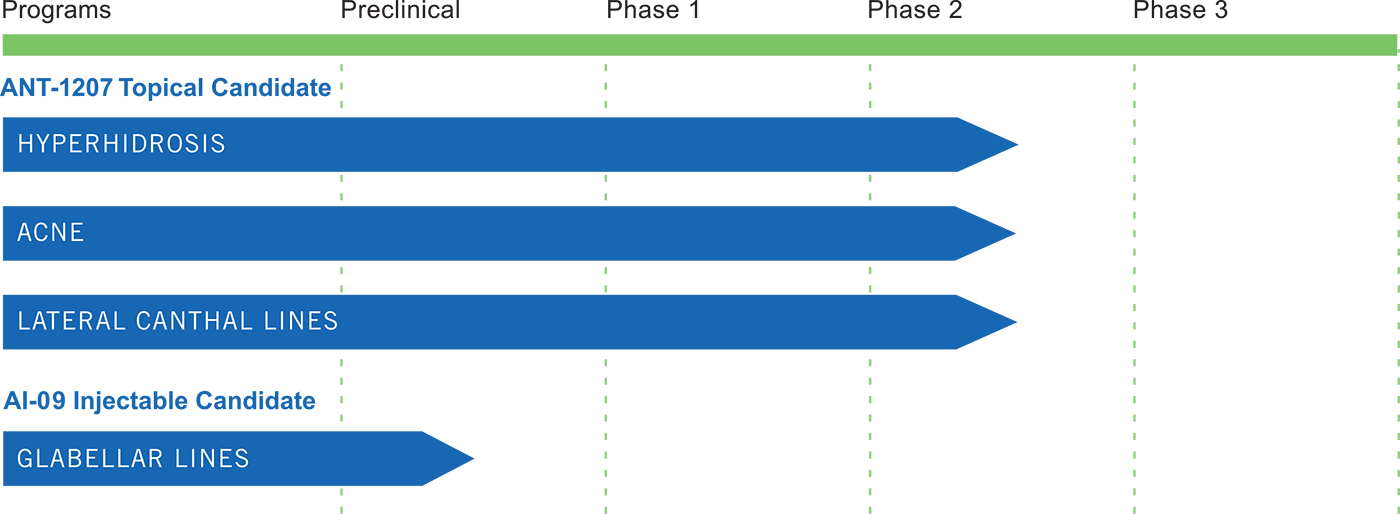
ANT-1207, Botulinum Toxin Type A Topical Lotion is an investigational product being developed for the potential treatment of hyperhidrosis (excessive sweating), facial wrinkles, and other FDA-approved clinical indications for injectable botulinum, as well as new potential clinical indications such as acne. The Company believes that this treatment has the potential to greatly expand the market for botulinum as it would overcome the limitations of injection, which include pain, bleeding and bruising as well as potential needle replacement.
ANT-1207 is a lotion that takes approximately ten minutes to apply in the physician’s office, where it is fully massaged into the skin. ANT-1207 incorporates a highly purified botulinum developed from Anterios’ proprietary cell line of C. Botulinum Type A.
ANT-1207 has been studied for the indications of primary axillary hyperhidrosis, facial acne, and lateral canthal lines (crow’s feet wrinkles), in multi-center, double-blind, randomized, controlled, dose-escalation studies that have enrolled approximately 500 subjects. These studies demonstrated that ANT-1207 was well tolerated and demonstrated clinically significant efficacy versus controls. It will be studied further in Phase 2b clinical trials.
AI-09 is a novel injectable formulation of Botulinum Toxin Type A that is packaged as a ready-to-use liquid preparation for therapeutic indications. AI-09 is a complementary product candidate to our topical botulinum product candidate ANT-1207, particularly for therapeutic indications where deeper delivery of botulinum is required.
AI-09 utilizes our proprietary NDS formulation technology, which when applied to injectable formulations, addresses several of the pharmaceutical issues with currently available injectable botulinum products. Currently, all injectable botulinum products on the market must be reconstituted in the physician’s office prior to use because they are lyophilized, or freeze-dried. They also contain human albumin, which presents a risk of transmittable disease. AI-09 utilizes neither lyophilization nor human albumin, and will be packaged as a ready-to-use injectable liquid in vials or pre-filled syringes. These enhancements produce an easier to use and more convenient product for the physician, and eliminate the risk of transmittable diseases that is associated with the inclusion of human albumin in currently marketed injectable formulations of botulinum. AI-09 incorporates the same highly purified botulinum used in ANT-1207. AI-09 is currently in preclinical testing.
Pipeline